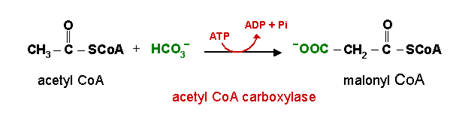
The key regulating enzyme of lipogenesis is acetyl-CoA carboxylase, which catalyzes the synthesis of malonyl-CoA from acetyl-CoA and CO2.
The activity of acetyl-CoA carboxylase depends on its phosphorylation status and its interaction with the antioncogen BRAC 1.
In its inactive form acetyl-CoA carboxylase is phosphorylated in serine, whereas the active form is not phosphorylated.
The phosporylation of acetyl CoA carboxylase is catalyzed by an AMP-dependent protein kinase (AMPK).
High AMP levels induce the phosphorylation and inactivation of acetyl-CoA carboxylase.
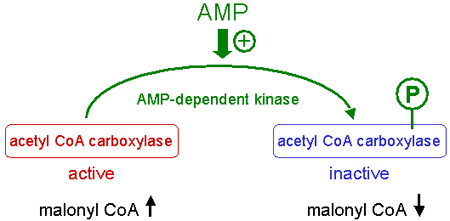
Therefore, low AMP levels activate acetyl-CoA carboxylase by inhibition of AMP-dependent protein kinase.
The activation of acetyl-CoA carboxylase leads to an increase of malonyl-CoA.
Tumor cells are characterized by high malonyl-CoA concentrations.
Malonyl-CoA itself inhibits carnitine acyl-CoA transferase-1, the enzyme that is responsible for the transport of fatty acids into the mitochondria, which leads to an inhibition of mitochondrial fatty acid degradation.
Under cell cultivation conditions the addition of extracellular AMP or AMP analogues, such as AICAR, into the medium of tumor cells induces an increase in fatty acid oxidation as well as a decrease in cardiolipin, triglyceride and cholesterol levels.
Cardiolipin is part of mitochondrial glycerol 3-P dehydrogenase and mitochondrial cytochrome C oxidase.
A decrease in cardiolipin leads to release of cytochrome C from the mitochondrial membrane, thereby activating procaspase 3 which induces apoptosis.